.
--> Overview of immune defenses
-->Immunity to Mycoplasma hyopneumoniae
.
OVERVIEW OF PORCINE IMMUNE DEFENSES AND IMMUNITY TO MYCOPLASMA HYOPNEUMONIA EINFECTION
By Dr. Romeo Sanchez, PhD, Associate Professor, College of Veterinary Medicine, University of the Philippines
……….
OVERVIEW OF IMMUNE DEFENSES
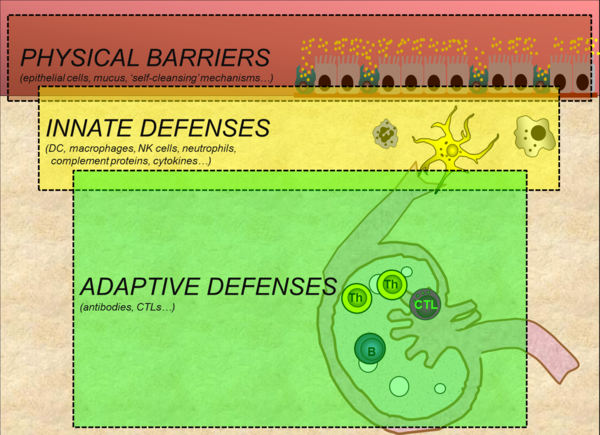
The pig relies on multiple defenses to protect itself against microbial invasion. The major defense systems are physical barriers, innate immunity and adaptive immunity (Figure 1). These distinct yet overlapping layers of defense interact extensively with one another and act collectively to protect the pig against infection and disease.
Figure 1. Three major defense barriers that protect pigs against microbial invasion (adapted from Tizard, 2013)
.
PHYSICAL BARRIERS
Physical barriers form the first line of defense against microbial invasion. The skin and the mucosal epithelia lining the respiratory, digestive and reproductive tracts form part of this system. Mucosal epithelia are further protected by a layer mucus containing antimicrobial molecules, and a variety of ‘self-cleansing’ mechanisms such as coughing, sneezing and mucous flow in the respiratory tract, vomiting and diarrhea in the digestive tract, and urine flow. These self-cleansing mechanisms serve to mechanically push-out microbes from these luminal surfaces.
In the respiratory tract, a vital ‘self-cleansing’ mechanism is the ‘mucocilliary escalator’; so-called because it likens the continuous movement of mucus along the surface of ciliated epithelial cells to a moving escalator. Inhaled microbes are trapped in the mucus layer and then transported via the ‘escalator’ to be coughed out or swallowed upon reaching the larynx. The tortuous structure of the turbinates in the nose of the pig is another respiratory barrier. Its unique conformation stirs turbulence in inspired air smashing and consequently trapping airborne microbes on the walls of the mucus- covered turbinate bones. The importance of these two barriers in the porcine respiratory tract becomes evident when these defenses are damaged by stress and/or infection with pathogens such as Mycoplasma hyopneumoniae and Bordetella bronchiseptica / Pasteurella multocida. In such situations, predisposition to infection and disease by other pathogens become common.
.
INNATE IMMUNITY
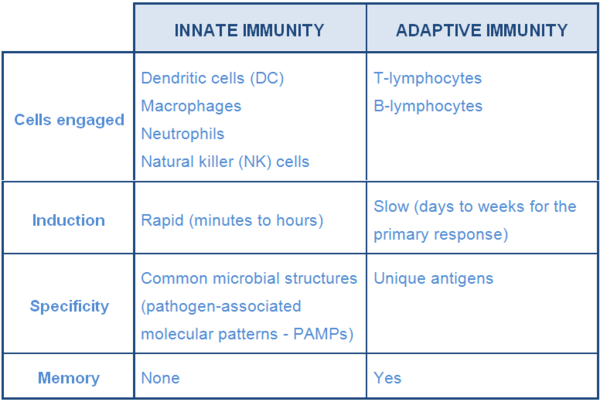
Microbes that are able to breach physical barriers are met by components of the innate immune system. This system is comprised of cells that detect and destroy invaders (dendritic cells, macrophages, NK cells, neutrophils...) and molecules such as those that promote inflammation (Table 1).
Table 1 Major characteristics of innate and adaptive immunity (adapted from Tizard, 2013)
Among the molecules of the innate immune system (IS) are pro-inflammatory cytokines and complement proteins. Cytokines such as interleukin-1 and TNF-alpha activate and attract phagocytic cells to the site of infection where they engulf, fragment and destroy invaders. Type-I interferons are another class of cytokines that although do not mainly promote inflammation, defend the host by making cells resistant to virus replication. Complement proteins are mainly enzymes whose activation leads to a cascade of reactions resulting to activation and attraction of defensive cells into the site of infection, and direct destruction of pathogens.
The innate IS typically responds within minutes to hours after contact with microbes. This response is preceded by an activation event involving PAMPs and pattern- recognition receptors (PRRs) such as toll-like receptors (TLR). PAMPs, molecular structures commonly found on or in pathogens, bind to corresponding TLRs on or in cells that are continuously patrolling potential sites of invasion (DC, macrophages, mast cells,…). This interaction between microbes and sentinel cells is regarded as a crucial point in the development of the immune response because it influences downstream events, more importantly, the activation of the next line of defense, the adaptive immune system.
Among the prerequisites for activation of the adaptive IS is the presentation of fragments of microbes (antigens) by patrolling DC and macrophages to lymphocytes of the adaptive IS. These patrolling ‘antigen-presenting cells’ (APC) of the innate IS migrate from the site of infection to draining lymph nodes or associated lymphoid tissues where they interact with lymphocytes initiating signalling pathways that trigger adaptive defenses.
.
ADAPTIVE IMMUNITY
Adaptive immunity is considered the ultimate and most potent line of defense against pathogens. Innate defenses can respond within minutes to hours after invasion, however, this response is short-lived and does not improve upon re-exposure to same pathogen (ie. no memory). In contrast, adaptive immunity takes some time to be activated (days to weeks) but can provide a longer duration of protection (weeks to years) which improves upon second and subsequent contact with the same pathogen. In this secondary response, the reaction is typically faster and more effective compared to the initial response. This rapid secondary response is due to the presence cells that has ‘memory’ of the first encounter. The adaptive IS can also recognize and respond to more pathogens (antigens) than the innate IS. This is because the cells of the adaptive IS, the B- and T-lymphocytes, possess a greater repertoire of receptors that allow them to recognize more antigens than cells of innate immune system which respond only to a limited set common microbial structures (PAMPs).
Adaptive defenses can be classified into two types: humoral or antibody-mediated immunity and cell-mediated immunity (CMI). Humoral immunity involves antibodies which are produced by differentiated B cells (plasma cells) upon stimulation by a combination of signals including antigen, T-helper (Th) lymphocytes that have been activated APCs, and cytokines produced by these cells. In the pig, there are 5 recognized (iso) types of antibodies (IgM, IgG, IgA, IgE, IgD). The type and amount (titer) of antibodies produced varies, with IgM being produced first followed by IgG during first contact with the antigen (primary response). During succeeding contact with the same antigen, IgG predominates and the total antibody titer is higher than during the primary antibody response. Antibodies are active against pathogens located outside of cells such as those that are free in the blood, in-between tissues and in secretions. A major function of antibodies is to stick to pathogens and block them from infecting cells (neutralization). Microbes bound by antibodies are also more easily captured and destroyed by phagocytic cells.
Cell-mediated immunity involves primarily cytotoxic T-lymphocytes (CTL). CTLs are activated by acombination of signals including cytokines (eg. interferon-gamma) and microbial peptides presented by MHC-I on the surface of infected cells. Activated CTLs survey the body for similar infected cells and selectively destroys them by forcing them to commit suicide (apoptosis).
During microbial invasion, either or both types of adaptive responses can be triggered. The type of adaptive response/s stimulated, its potency and duration are influenced by many factors. Among these are the type and amount of pathogen entering the pig, and the interactions and outcome of interactions between the microbe and immune cells (APCs and lymphocytes). In vaccination, the aim is to mimic immunity induced by natural infection with the wild-type organism. With killed vaccines, adjuvants are added to enhance the interaction between vaccine antigen/s and cells of the IS with the purpose of triggering, ideally, both antibody and cell-mediated responses of sufficient potency.
Adaptive defenses (antibodies and immune cells) can be transferred from dam to offspring via ingestion of colostrum and milk. Indeed, this passively transferred immunity is largely responsible for providing protection to piglets a few days to weeks after birth and is among the reasons for vaccination/exposure of gilts and sows prior to farrowing. As this maternally-derived passive immunity eventually wanes at around the time of weaning, antibodies and immune cells actively produced by the piglet assumes protective cover.
.
IMMUNITY TO MYCOPLASMA HYOPNEUMONIAE
Mycoplasma hyopneumoniae (M. hyo)causes enzootic pneumonia, a chronic disease of pigs characterized by coughing and reduced production performance. M. hyo binds to ciliated epithelial cells along the respiratory tract but does not go deeper into the lung. Pathology results from damage to cilia and epithelial cells which can lead to disturbance in mucosal clearance, and consequently, predisposition to infection and potential exacerbation of disease by other pathogens. Peri-bronchiolar infiltration of mononuclear cells, hyperplasia of associated lymphoid tissues, and accumulation of inflammatory cells in alveolar spaces restrict air movement contributing to pathology and clinical disease.
The exact mechanisms of protection following infection with M. hyo are not yet fully understood although both antibodies and CMI are thought to be involved. Mucosal IgA antibodies are thought to bind the microbe, prevent it from engaging receptors on cilia, and promote their phagocytosis by alveolar macrophages. The role of systemic antibodies, however, is still unclear since serum antibody does not correlate with degree of protection. Prior to specific activation of the adaptive IS, M. hyo can activate macrophages, NK cells, complement and lymphocytes in a non-specific manner. These immune reactions induced by M. hyo infection are important in host defense, but have also been suggested to be involved in the development of lesions and exacerbation disease induced by Mycoplasma.
The precise protective mechanisms after vaccination are also not yet fully understood. Vaccinated pigs have been shown in several field and experimental studies to have better production performance and/or lower clinical and lung lesions scores compared to non-vaccinated pigs, but the exact protective mechanisms have yet to be defined. Similar to natural infection, antibody, and perhaps more prominently, cell-mediated responses, are thought to be involved. In vaccinated pigs, evidence suggesting higher CMI activation and response has been reported. Among the suggested reasons for the better performance of vaccinated pigs are lower numbers ofM. hyoand infiltrating immune cells in the lungs, and lower levels pro-inflammatory cytokines compared to non-vaccinated pigs.
.
This article appeared in Axis - VIV 2013 ©Copyright 2013, All Rights Reserved.
.
<< Back to Disease Informations
Related topics: M. hyo immune defense ep enzootic pneumonia disease information swine mycoplasma hyopneumoniae

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam