.
--> Introduction
--> Conclusion
.
COGLAPEST®: COMPARISON WITH TWO MODIFIED LIVE VACCINES CONTAINING A CHINESE STRAIN
By Dr Eric Brunier, Regional Market Manager – Swine, Ceva Animal Health Asia Pacific
With collaborators: Dr. Achinee RUNCHAROEN - Market Manager Swine of Ceva Animal Health Thailand
and Dr. Renato BIJASA - Market Manager Swine of Ceva Animal Health Philippines
.
INTRODUCTION
As described in the article concerning the possibility to use a mass vaccination program with COGLAPEST® in a sow herd, Classical Swine Fever (CSF) also called Hog Cholera is such a threatening danger for a farm that the vaccination of the breeders and of the pigs is de facto compulsory. Different vaccine strain, included in different commercial vaccines, are present on the market. Their efficacy and sefety, however, are not identical. Before choosing the vaccine to use, it is important to consider these facts.
In Asia, CSF is an enzootic disease in all the countries with a swine population but Japan. In enzootic areas, only modified live vaccines (MLV) can bring the expected efficacy. Inactivated vaccines (sub-unit vaccines) are kept for free areas in case new outbreaks occur. Among the MLV, different strains exist as Chinese strains, proceduced by lapinization or in cell cultures, gpE-strain, K-LOM strains and Thiversal strain. This last strain belongs to Ceva Animal Health and is present in the vaccine COGLAPEST®. For more than 20 years, it has long shown in the Asia market its strength and efficiency, its long lasting protection, its exceptional potency as well as its outstanding safety. It became, for these reasons, the first choice in commercial farms in the Philippines, in Thailand and in Vietnam.
The objective of the article is to show two different trials that have tested, in field conditions, the immunogenicity of COGLAPEST® versus two different commercial MLV, both of them containing a Chinese strain.
In order to define the protection level conferred by the vaccine, the reference method is the challenge test after vaccination. This test is not adapted to be used in commercial farms under field conditions. Fortunately, concerning the vaccination against CSF, the protection conferred by the vaccine is well correlated with the level of neutralizing antibodies, measured preferentially by serum-neutralization test (the reference test). This indirect method allows the comparison of vaccines under field conditions.
.
TRIAL IN THAILAND
The first trial was done in Thailand in a farrow-to-finish farm that was using a gpE-strain MLV on sows during the gestation. Two groups of 30 piglets each have been constituted at weaning and raised side by side. Each group was vaccinated twice by an intra-muscular injection of 2 ml of a commercial MLV: at 5 and 7 weeks of age. The first group (G1) was vaccinated with COGLAPEST®; the second group (G2) was vaccinated with a vaccine containing a cell-cultured Chinese strain. Blood samples were taken at 5, 7 and 14 weeks of age. The antibody level against CSF has been analysed by serum-neutralization test.
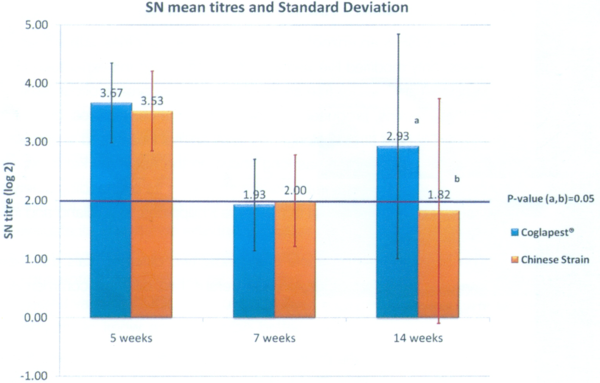
At five weeks of age: The antibodies measured are still the maternal derived antibodies (MDA). The vaccination, done the same day as the blood samples, was not able to develop any immunity yet. There is anyway a significant difference between G1 and G2 (p value = 0.008 with T test). It is difficult to compare both groups if, before the vaccine take, there is a difference between each group already. Why should there be a difference? The antibody level heterogeneity may be linked to a heterogeneity in the sows’own antibody level at farrowing or because some piglets didn’t drink the colostrum in a satisfying way. Considering that the sow vaccination had been correctly done during the gestation, the most probable explanation was the colostrum consumption. In order to avoid this bias, the piglets with a low antibody level (<3 SN log 2), which are susceptible to not having drunk enough colostrum, have been taken out of the trial. The results of the groups, resulting from this correction were: G1: 3.67; G2: 3.53; p = 0.50 (Graph 1). The groups are now perfectly comparable. The following results will concern those modified groups.
Graph 1 Immune response comparison between COGLAPEST® and a cell cultured Chinese strain vaccine with vaccination at 5 and 7 weeks of age
At what age could we do the vaccination? At five weeks of age, the geometric mean is 3.55 (for all piglets of both groups) and the standard deviation is 0.65. The MDA level is lower than the inactivation threshold of 5 (1:32) and higher than the protection threshold of 3 (1:8). This timing of five weeks of age for the first vaccination is perfectly adapted.
At seven weeks of age: Both groups are still comparable with an antibody level of 1.93 for G1 and 2.00 for G2 (p=0.93) (Graph 1). We observed that two weeks after vaccination the antibody level has decreased when compared with the level at five weeks. That is normal. The MDA have continued to decay while the antibodies due to vaccination have not reached yet the level the MDA had at five weeks. For CSF vaccination with a MLV, we expect an increase of antibodies up to five weeks after vaccination (10 weeks of age in that case). Concerning the protection, we expect a protection by the vaccine with an antibody level from 2 SN log2. Both groups are at the protection threshold.
At 14 weeks of age: There is a significant difference between both groups. G1 has an antibody level of 3.93 while G2 has a level of 1.82 (p=0.05) (Graph 1) with a more important standard deviation. Group 2 is in average under the vaccine protection threshold; while Group 1 has an antibody level that has increased far over this protection threshold. If we consider the ratio of piglets that are over that limit, it is 75% for G1 and 53% for G2.
Conclusion: Compared in field conditions with a commercial MLV containing a cell-cultured Chinese strain, COGLAPEST® has shown at the same time a significant difference in the level of antibodies and a superior ratio of piglets over the defined protection threshold. We may expect a better level of protection with COGLAPEST®.
.
TRIAL IN THE PHILIPPINES
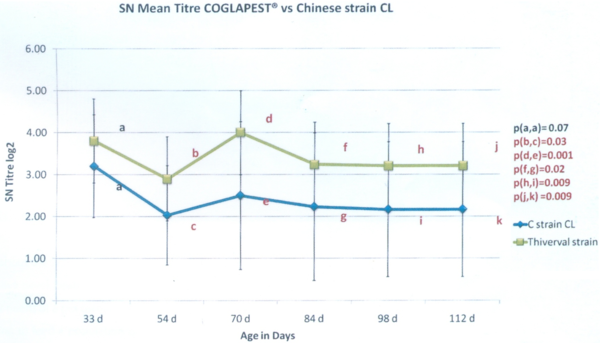
The second trial was done in a 1.500 sows farrow-to-finish farm. The material and method is quite comparable with the first trial presented above. In this farm, the sows are vaccinated 4 weeks before farrowing with a commercial MLV containing a CL-Chinese strain. Two groups of 30 piglets each have been selected at weaning and have been raised side by side. They have been vaccinated at 5 and 8 weeks of age with a commercial MLV vaccine against CSF: Group 1 (G1) was vaccinated with COGLAPEST® while Group 2 (G2) was vaccinated with the same vaccine used on the sows. Blood samples were collected at 33, 54, 70, 84, 98 and 112 days old. The antibody level was measured by serum-neutralization test. The following results are expressed in SN log2.
At five weeks of age: As for the trial, 5 weeks of age appeared to be a good timing for the vaccination considering that the animals had a MDA level under the inactivation threshold of 5. Moreover, there is no significant difference in the MDA level between both groups before vaccination.
Graph 2: SN titration results
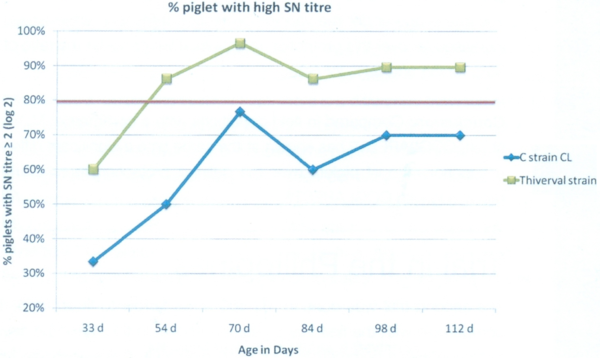
From 54 days old until 112 days old: (Graph 2) We observed a statistically significantly higher level of antibodies due to vaccination on G1, vaccinated with COGLAPEST®. When we consider the ratio of piglets with an antibody level higher than 2 SN log2, it is all the timer higher than 80% for G1 while it is lower for G2 (Graph 3).
Graph 3: Percentage of piglets with SN titre over 2
.
CONCLUSION
In both trials, in comparison with two different commercial MLV containing Chinese strains, COGLAPEST® has triggered the synthesis of a significantly higher level of serum-neutralizing antibodies after vaccination and the number of piglets with an antibody level higher than 2 SN log2 was systematically higher. COGLAPEST® may provide a better protection to the vaccinated animals.
.
(Source: "Axis Issue 10 - APVS & VIV Asia - Special Edition" - March 2011)
.
<< Back to Classical Swine Fever - Hog Cholera
<< Back to Disease Informations
Related topics: csf comparison experiment classical swine fever swine vaccine coglapest® trial protection vaccination

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam