.
--> Introduction
--> Results
--> Discussion and Conclusions
.
COMPATIBILITY OF COGLAPEST® AND COGLAPIX® VACCINES IN PIGS
By Vilmos Palya1, István Kiss1, Roman Krejci2
1Ceva Sante Animale, Scientific Support and Investigation Unit, Ceva-Phylaxia Ltd., Budapest, Hungary,
2Ceva, Libourne, France.
.
Introduction
Porcine pleuropneumonia is a highly contagious respiratory disease caused by Actinobacillus pleuropneumoniae (A.p.). The disease occurs worldwide with an increasing incidence. Controlling the disease presents a challenge, for its rapid onset and persistence in infected herds, and because of the issue of antibiotic resistance. Vaccination, however, can provide efficient protection against the disease by decreasing the prevalence and extension of pneumonia and pleuritis and as a result animals with better weight can be delivered to the market.
Classical swine fever (CSF) is a highly contagious disease of pigs with the potential of devastating epidemics, which also occurs widely in some parts of the world. Outbreaks of CSF are controlled by strict measures, such as culling and movement restrictions, which, besides the direct consequences of the disease, have severe impact on the pig industry. In countries where the virus is endemic, vaccination is used to prevent the spread of the virus and the consequence of infection.
Based on practical and economic considerations the simultaneous vaccination of piglets against several diseases is desired, provided that the efficacy of each vaccine is not compromised. Upon the demand of vet practitioners the study was conducted in order to test the compatibility of Coglapix® and Coglapest® vaccines, when the latter was reconstituted in Coglapix®.
.
Materials and Methods
Six weeks old pigs were vaccinated twice, three weeks apart, either with Coglapest® or Coglapix® or combined vaccine of Coglapest® and Coglapix®. The efficacy of Coglapix® was tested by challenging the pigs with an A.p. serotype-2 strain via aerosol in a dedicated chamber by applying approximately 108 CCU/pig of the bacterium suspension. Parameters related to immunity (humoral immune response and protection against A.p. challenge) were compared between the vaccinated and control pigs.
APX-2 ELISA (in-house assay) and Chekit APX-4 ELISA (Idexx) was used to measure antibody response to Coglapix® and neutralising peroxidase-linked assay (NPLA) was used to measure antibody response to Coglapest®.
Scoring of lung lesions was performed according to Hannah et al., 1982., and the efficacy of Coglapix® vaccine was calculated according to Jones et al., 2005.
Serological results, body weight gain, and lung lesion score data were analysed by ANOVA. Differences were considered significant at p<0.05.
^ Top page
.
Results
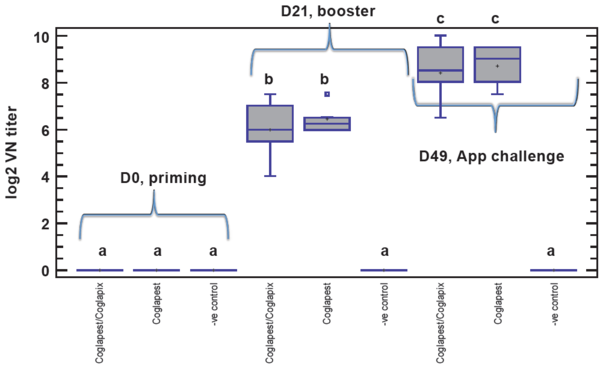
Humoral immune response to Coglapest® did not differ significantly between the Coglapest®/Coglapix® combined and Coglapest® alone vaccinated groups (Figure 1).
Figure 1 Box-and-Whisker plot of antibody response against CSFV
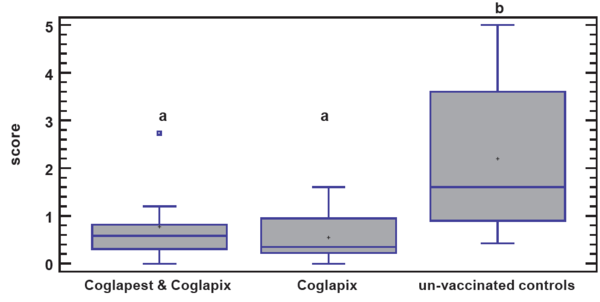
Following challenge with A.p. serotype-2 strain, 20% of the unvaccinated control pigs died, while no mortality in the vaccinated groups was recorded. The two vaccinated groups had significantly higher mean body weight gain than the unvaccinated control group, but did not differ significantly from each other. Further, the mean pathological scores were significantly higher for the non-vaccinated control group than for the two vaccinated groups, which did not differ significantly (Figure 2).
Figure 2. Box-and-Whisker plot of the lung lesion score data of the groups following A.p. serotype 2 challenge.
^ Top page
.
Discussion and Conclusions
Pigs vaccinated with Coglapest® either alone or in combination with Coglapix® equally seroconverted and produced very similar amount of CSFV VN antibodies both after the primary and also after the booster vaccination.
Vaccination with Coglapix® either alone or in combination with Coglapest® induced high level of protection against the challenge with the virulent strain of A.p. serotype 2 without significant difference between the two vaccination regimens.
^ Top page
.
References
Jones, G.F., et al., J. Swine Health Prod. 2005; 13(1): 19–27.
Hannan, P., et al., Res. Vet. Sci. 1982. 33: 76-88.
.
(Source: Axis Issue 02 / Sept 2013 - Ceva Asia Pacific - Proceedings APVS 2013)
.

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam