...
--> Introduction
--> Characteristics of incubating eggs, including interactions with the holding conditions
--> Physical quality of the egg
--> Embryo development at oviposition
--> Time and conditions between oviposition and storage
--> Storage of incubating eggs
--> Ventilation and carbon dioxide concentration
--> Effects of storage time and turning during incubation on hatching time and chick quality
--> Spreading of hatching time, hatchability and its relation to chick quality
--> Conclusion
--> References
...
THE DAY-OLD CHICK: A CRUCIAL HINGE BETWEEN BREEDERS AND BROILERS
By E.DECUYPERE, K. TONA, V. BRUGGEMAN and F. BAMELIS, Laboratory for Physiology and Immunology of Domestic Animals - Department of Animal Production, Faculty of Agricultural and Applied Biological Sciences - K.U. Leuven, Kasteelpark Arenberg 30, B-3001, Leuven, Belgium
Keywords: Chick quality; egg characteristics; hatchability; incubation conditions
.
INTRODUCTION
The quality of day-old chicks is uppermost in the minds of most industry poultry managers. Hatching success and the quality of the chicks produced have traditionally been the yardsticks by which a hatchery or the incubators in it are judged. Hatching success and chick quality have been and are still often linked to each other, leading to the idea that, if hatchability has been maximised, chick quality is also automatically optimal. But is maximal hatchability the best indicator for the highest post-hatch viability, growth and the most efficient feed conversion? Assuming that maximal hatchability is an absolute prerequisite for hatching success, is it synonymous with the highest chick quality? The question concerning the conditions in the incubator can be formulated in another way: how much variation, if any, is there in the conditions within the zone of incubation that guarantees optimal hatchability in order to obtain the “highest chick quality”?
A 1-day-old chick of good quality must be clean, dry and free from dirt and contamination, with clear and bright eyes, free from deformities, with a completely sealed and clean navel. No yolk sac or died membrane should protrude from the navel area. The body should be firm to the touch, and there should be no signs of respiratory distress. The chick should be alert and interested in its environment, responding to sounds. The legs should have the normal conformation, with no swelling and no hock or skin lesions; the beak should be well formed and the toes firm and straight (Funk and Irwin, 1955; Raghavan, 1999).
At the outset in this paper it will be assumed that maximising hatchability automatically implies optimising chick quality. To test this hypothesis, three groups of factors that affect hatchability and chick quality will be discussed:
(1) The characteristics of incubating eggs;
(2) The incubation conditions;
(3) The conditions prevailing between hatching and the placement of the birds on the broiler farm.
It must be appreciated that these groups of factors should not be seen as being independent of each other. For example, the conditions existing during incubation may interact with the quality of the eggs set or the holding time; the conditions after hatching and before the placement of the chicks on the farm may depend on the time taken to hatch, the time over which the hatching period is spread and, hence, on the egg holding time or the age of broiler breeders.
Therefore, although this overview will focus on incubation as a crucial step in the production of day-old chicks of high quality, two groups of factors that affect the quality of day-old chicks will be described briefly here. It is assumed that all the factors that constitute good broiler breeder husbandry and are important for the production of eggs with good hatchability are fulfilled, i.e. the genetic make up of the stock (Crittenden and Bohren, 1962), the housing conditions, the feed and water requirements of the breeding birds during growth and production, the age of the flock, its health, and the management of the male birds.
.
CHARACTERISTICS OF INCUBATING EGGS, INCLUDING INTERACTIONS WITH THE HOLDING CONDITIONS
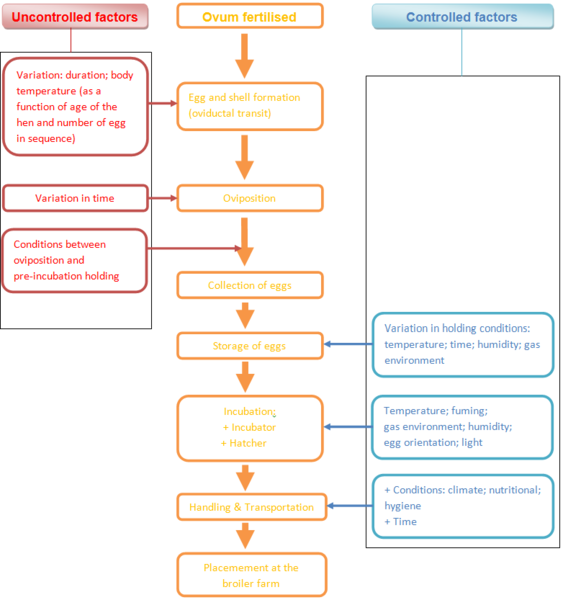
Between the fertilization of the ovum (the starting point of embryo development) and the placement of the day-old chick at the broiler farm, many factors affect the development of the chick and determine its final quality. Some of these can be controlled while others cannot (Figure 1). Not all these factors are environmental. There is also a substantial endogenous or genetic component that contributes to the variability in the developmental parameters and may also interact with environmental variables.
Schema 1 Scheme of factors affecting chick quality from fertilisation of the ovum to placement of the day-old chick at the broiler farm
In the chronological order of the developmental process from the point of fertilisation to the start of incubation, the physical quality of the egg, the stage of development of the embryo at oviposition, the time taken and the conditions prevailing between oviposition and storage and conditions of incubating eggs all need to be considered.
^ Top page
.
PHYSICAL QUALITY OF THE EGG
The quality of the egg embraces its size, shape, colour, cleanliness, the integrity of the shell and the absence of shell malformations. Egg size is affected by a number of management and nutritional factors but, of course, also by the age (Crittenden and Bohren, 1962; MacLoughlin and Gous, 1999; Vieira and Moran, 1999) and genotype of the breeder parents. The size of the egg is important because of its direct relationship with the size of the day-old chick (Moran, 1990) which comprises 64 - 70% of the weight of the egg (Merrit and Gowe, 1965). Egg shape and colour are highly heritable and affect the number of eggs that hatch.
Egg size and shape, together with shell porosity; largely affect the water loss at a given humidity during incubation (Taylor, 1999). Size may also influence the heating or cooling requirements during the last week of incubation (Deeming, 1996). When the embryo has a substantial mass, all heat has to be dissipated through the surface of the egg. The physical characteristics of the egg may therefore interact with or influence the conditions requires for optimal incubation.
Egg inclusions such as blood, blood spots or meat spots are not known to influence hatchability. Cleanliness of the shell is not simply a good indicator of the management standard on the breeder farm. It is also likely that hatchability will suffer from shell contamination or that chick quality and viability will be poor as a result of the newly hatched chicks carrying a burden of pathogenic organisms within their respiratory or alimentary tracts. Washing and disinfecting eggs for incubation are, of course, possibly preventative measures that may be instituted, but it should be realized that they can introduce additional effects, such as cuticle removal, which can affect hatchability (Peebles and Brake, 1986).
^ Top page
.
EMBRYO DEVELOPMENT AT OVIPOSITION
Variations in the stage of development at the moment of oviposition have been found in a number of laboratories (Sturkie and Williams, 1945; Butler, 1991). Embryonic development at oviposition was found to be different in eggs from different genetic lines, probably as a direct effect of the genetically determined aped of early cell division and development, as well as in eggs from parents of different ages. The latter may be indirectly linked to variations in oviductal transit time and/ or body temperature and is likely to consist of genetic and environmental components (Decuypere and Michels, 1992; Shanawany, 1992).
Embryos at the pre-gastrula stage at oviposition were less able to withstand prolonged storage than those at the gastrula stage (Decuypere and Michels, 1992; Wilson, 1991). Because heat treatment for one of a few hours daily before and during egg storage may benefit hatchability in lines of chickens which normally exhibit a rather low hatching percentage (Decuypere and Michels, 1992), it may be speculated that these lines lay their stage of blastoderm development.
^ Top page
.
TIME AND CONDITIONS BETWEEN OVIPOSITION AND STORAGE
An egg should have cooled down to 27oC about 6 hours after being laid. When the ambient temperature is high (above physiological zero), slow cooling of the eggs could cause a problem as a result of slow cell multiplication and abnormal embryos. This situation occurs if eggs are not collected frequently (eggs should be collected more frequently on warm days) and are kept warm in nests by the hens sitting on them and/ or by the nature of the nesting material (Meijerhof, 1994).
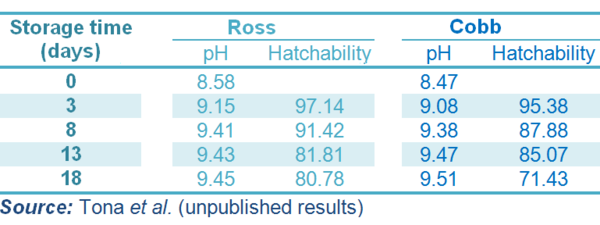
Table 1 pH values and hatchability percentage of incubating eggs of Ross and Cobb strains according to storage time
At oviposition the egg contains a high concentration of carbon dioxide which stars to escape after laying and during storage, leading to a rise in the pH of the albumen. This is important because early developmental activity is controlled by pH-dependent enzymes. Excess carbon dioxide loss causes the albumen to have an excessively high pH and this is negatively affects the initiation of embryo development. If the loss of carbon dioxide is too low, the pH of the albumen will also be too low resulting in eggs which are “too fresh” and not hatch as well as those stored for 3-4 days (Table 1). This process of carbon dioxide loss is also temperature-dependent and may be stimulated by cooling after oviposition (Lapao et al., 1999; Tazawa and Whittow, 2000).
^ Top page
.
STORAGE OF INCUBATING EGGS
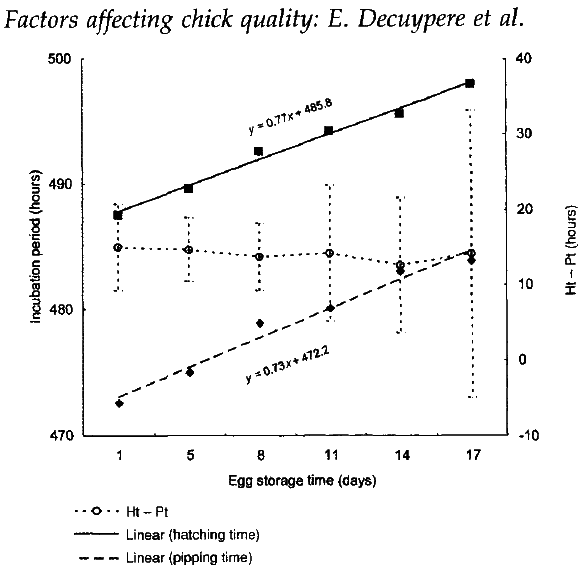
Storage of eggs is normal practice after their collection and often a necessity in commercial incubation. It is, to some extent, influenced by the desire to avoid mixing eggs from different breeder parents, from different ages of flock, or from flocks with a doubtful health status. However, storage results in longer incubation times; for examples, storage for one extra day adds one hour to the incubation time (Mirosh and Becker, 1974; Muambi et al., 1980; Figure 1). Longer periods of storage will therefore increase the spread of time over which hatching takes place and this may influence the total hatchability (Muambi et al., 1982), as well the overall quality of chicks.
Figure 1 Hatching time, pipping time and intervals between hatching and pipping times (Ht-Pt) in relation to pre-incubation storage time of eggs below 12oC and 90-95% humidity.
A critical factor in storage, besides humidity, is the temperature. Current recommended values range from 13oC to 17oC. In any case, the temperature at which pre-incubation eggs are held must be below that of the threshold for development. There is no consensus as to what constitutes this physiological zero, but as discussed in a review by Decuypere and Michels (1992), it is considered to lie within the range 19-28oC. Under practical conditions there appears to be an empirical relationship between storage time and temperature: short-term storage benefits from the higher temperatures prevailing (15-16oC) and storage times in excess of 5 days benefit from lower temperatures (11-12oC). However, if the minimal value for the physiological zero is at least 19oC, why is there the practical need for a much lower temperature when eggs are stored for longer periods? These findings suggest that some particular, but not global or proportionate, development can take place at so-called sub-threshold temperatures. If this disproportionate development progresses beyond a certain stage, it may interfere with the embryo viability (Decuypere and Michels, 1992). This may also be linked to the observation that periodic heat treatment during long periods improves hatchability because it allows the embryo to regress disproportionate development (Wilson, 1991). Moreover, prolonged storage may interact with the developmental stage at oviposition, as mentioned earlier, thereby giving better or worse results depending on the strain or age of the breeder parents or the environmental conditions. These interactions are currently under further study in our laboratory.
^ Top page
.
INCUBATIONS CONDITIONS
A wealth of published material exists about single- and multi-stage incubation. Apart from the prophylactic aspect, the results should be better with single-stage incubation which, it is frequently claimed, enables a specific group of eggs to be provided with so-called optimum incubation conditions. The fact that they are not suggests that the knowledge of the detailed requirements of an egg at different stages of incubation is incomplete or that insufficient is known about the many factors (e.g. genetics, parental age, egg quality, storage conditions) that interact with incubation conditions.
^ Top page
.
INCUBATION TEMPERATURE
The environmental temperature for the highest hatchability lies within the range 37-38oC, values which are based on the famous bell-shaped curve of Barott (1937). The equation indicates that the optimum temperature, which has been applied since then, is 37.8oC. The question remains, however, as to how narrow this temperature zone may or must be in order to obtain an optimal hatch? The answer to this question has practical implications on the temperature gradients that occur in the setter machines, and how these are affected by such factors as their size, the way they are built and equipped, the position of the trays, the ventilation rate and the spaces between the eggs. It is often suggested that the temperature should not be allowed to vary by more than 0.3oC from the optimum, thus determining the upper and lower limits of the optimal incubation temperature. However, the tolerance to deviations in temperature from the standard of 37.8oC is a function of the duration of exposure to these deviations (Thompson et al., 1976) and of the stage of the development. Some periods were found to be more and others less sensitive (Ande and Wilson, 1981). The tolerance is found to be higher for temperatures below 37.8oC than for those above it. Strain and line differences also affect the tolerance to variations in the standard temperature and to temperature fluctuations occurring during incubation (Thompson et al., 1976; Ande and Wilson, 1981; Decuypere and Michels, 1992). From these results it can be concluded that selection in the domestic fowl for egg production compared with meat production can alter the optimal range of incubation conditions with respect to temperature and other factors.
The next question to be asked (and already formulated in the introduction) is whether the temperature that produces the best hatchability also automatically results in chicks of the highest quality. Bearing in mind that, during embryogenesis, neither the growth of the body nor the development of its functional systems is identical for all its components, then deviations in temperature during incubation and, hence, in the rate of development of the embryo, which is time limited, may alter proportional growth. This may also influence functional processes of the embryo and the chicken in a differential way, depending on the period when this temperature deviation is applied. Examples of this may be found in the following themes:
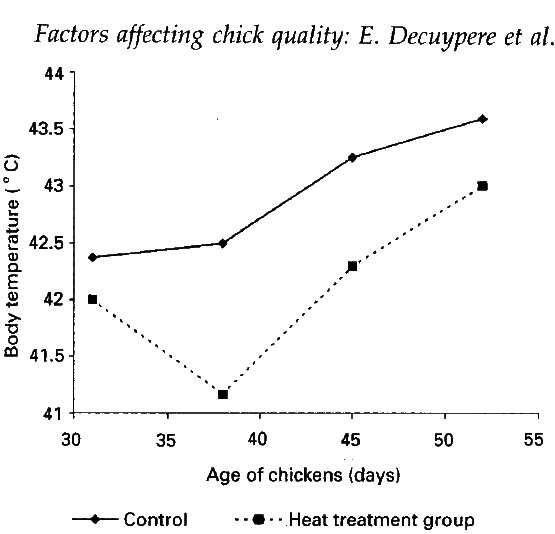
- Temperature and thermoregulation: the incubation temperature has been found to affect postnatal body temperature (Figure 2) and hence during thermoregulatory ability. Exposure to a low temperature (33.5oC) during the last days of incubation affects post-hatch heat production (Decuypere, 1984; Minne and Decuypere, 1984).
Figure 2 Effect of 3 hours of daily heat treatment at 39oC from days 11 to 20 of incubation on postnatal body temperature by exposing 6-week-old birds for 4 hours to temparature below 40oC and 80% relative humidity. Source: Meijerholf (unpublished results).
- Temperature and endocrine development (e.g. stress susceptibility): in the chick embryo periodic exposure to cold during days 13-19 of incubation changes the subsequent reaction to stress 8 weeks after hatching (Avrutina and Kisljuk, 1982; Avrutina et al., 1995) by changing post-hatch endocrine functions (Nvota et al., 1980; Decuypere et al., 1988). Heat treatment during incubation decreased plasma thyroxine (T4) and corticosterone concentrations in birds 40 days after hatching (Iqbal et al., 1989; 1990).
- Temperature and growth: incubation temperature is important not only for hatchability but also for postnatal growth (Romanoff, 1935; 1936). Periodic exposure to a low temperature (33.5oC) during the last quarter of incubation stimulated postnatal growth (Decuypere et al., 1979; Nvota et al., 1980; Kuhn et al., 1982). Whilst periodic heat treatment during the first 10 days or the last 10 days of incubation (39oC) improved feed efficiency, body weight was not affected (Abd El Azim, 1991).
The big challenge is that hatchability must not be altered or only minimally so.
^ Top page
.
VENTILATION AND CARBON DIOXIDE CONCENTRATION
According to Brian (2000), a carbon dioxide concentration of 0.1-0.4% is optimal in a multi-stage setter (this represents a tolerance range of 300 %!!). It rises from 0.5% to 0.8% in the hatchers (a range of 60%), close to the limits of liveability for chicks. Some evidence suggests that better results can be achieved in single-stage setters if the concentrations of carbon dioxide during the early stages are 0-0.6% (Tazawa, 1980a; Tullett and Burton, 1986; Okuda and Tazawa, 1988). It is believed that this concentration acts as a stimulant to early embryonic development, but it may also slightly increase the pH during these early stages (Tullett, 1990), thereby stimulating some enzyme activities during this period.
Within rather narrow stimulating some enzyme activities during the entire incubation period are optional, although it must be borne in mind at all times that high concentrations will jeopardise the liveability of the embryo (Tullett, 1990). There is an obvious lack of knowledge in this area and regulation to attain optimal carbon dioxide concentrations is still considered a problem in practice.
^ Top page
.
EFFECTS OF STORAGE TIME AND TURNING DURING INCUBATION ON HATCHING TIME AND CHICK QUALITY
Empirically, it was found as an interspecies relationship that:
I = 12 X egg (0.22)
and I X MO2 = c X egg
Where:
- I = period of incubation,
- egg = mass of the freshly laid egg in g,
- MO2 = oxygen flux in ml/day and
- c = a constant.
This indicates that, for a given egg mass, an egg which consumes less oxygen at the plateau stage needs a longer incubation period. If this relationship is further confirmed within a species, it may have the following consequences:
- If the MO2 at the plateau stage is matched to the shell conductance then, for a given egg mass, an egg with a lower shell conductance needs a longer incubation time (Okuda and Tazawa, 1988; Tullett, 1990; Ancel and Visschedijk, 1993).
- Because prolonged pre-incubation decreases the MO2 (Haque et al., 1996), a longer period of storage (1, 2 or 3 weeks) results in a longer incubation time.
The results of Haque et al. (1996) illustrate the relationship between storage and MO2 at the plateau phase. The fact that a longer storage time results in a decrease in the T3 concentrations at hatch (Muambi et al., 1982) may be linked to the longer incubation time (T3 is an important stimulus of hatching). It also strengthens the hypothesis of a lower MO2 at the plateau phase as a function of storage time, because the MO2 is related to thyroid hormone maturation.
In the chicken the critical period for lack of egg turning ranges from day 3 to day 7 of incubation (Deeming, 1990; 1991). Failure to turn eggs may have adverse effects on gas exchange through the chorioallantois; the unabsorbed albumen is interposed between the chorioallantois and inner shell membrane, hence reducing the gas exchange, decreasing the arterial oxygen pressure (paO2) of late embryos and increasing haematocrit values (Deeming, 1989a and b; Wilson, 1991). Also, the MO2 of unturned eggs is lower (Tazawa, 1980b). All this may result in an increase in the length of the incubation time and decreased hatchability. Even if the eggs do hatch, there is an increased susceptibility during later development of the birds contracting ascites and rounded heart disease. The relationship between the length of the incubation time and the other parameters associated with incubating has also been substantiated in embryos of strains sensitive to ascites (Buys et al., 1998).
^ Top page
.
SPREADING OF HATCHING TIME, HATCHABILITY AND ITS RELATION TO CHICK QUALITY
A slight delay in hatching time or a more pronounced spreading of it may affect (and decrease) hatchability in a fixed management schedule where the hatching is judged after exactly 21 days. Spreading of the hatching period, with a view to optimising the quality of the day-old chicks or chick placement, may gain even more attention now because of the well recognised fact that any delay in food and/ or water intake (although food is much more important than water) after hatching will negatively affect a number of performance parameters. Hatched chicks can rely on the residual yolk sac, although the use of the yolks is retarded when they are fasted (Vieira, 1999). The development of the gastrointestinal system is stunted under fasting conditions, and this may be related to the retarded utilisation of the yolk (Dibner, 1999). The metabolism is lower, a delay occurs in the maturation of the enzymatic systems that control metabolism (e.g. the deiodination system and the activation of the T3 pathway), and the development of the immune system may be retarded. Furthermore, the uptake of IgG, provided by the yolk, during the first day after hatching will also be slower (Dibenr, 1999).
As chicks are precocial, they will forage for feed almost immediately after hatching and begin to grow, whereas holding them without feed results in decreased body weight and a decrease in the overall performance of the broiler (Noy and Sklan, 1999).
Under practical conditions many birds have access to feed only at 36 - 48 hours after hatching, and during this time body weight decreases quickly (Noy and Sklan, 1999; Pinchasov and Noy, 1993).
Firstly, there is the hatch window (24 - 36 hours) or the spread between late and early hatchers. This can be influenced by the homogeneity or otherwise heterogeneity of the starting material (the incubating eggs); for example, eggs from old breeders hatch earlier than those from young flocks and chicks from smaller eggs (within a flock age) hatch earlier than those from large eggs. This points immediately to the independent effects of the size of the eggs and the age of the breeding birds, although young breeders do lay smaller eggs. Mixing eggs from different storage conditions or times, and variations within the incubator (e.g. temperature gradient) can affect the size of this spread. A spread in the hatching period will increase the numbers of chicks that will be forced to do without food or water for longer times.
Secondly, the time spent in the hatchery and the time taken to transport the birds to the farm (together with the conditions during transportation) can involve a further holding period and a delay in access to food and water. Moreover, an interaction between the time delay from hatch to access to feed and water and egg size seems to occur. Small chicks have to be placed and fed as soon as possible after hatching as they have a proportionally smaller residual yolk.
^ Top page
.
CONCLUSION
It can be stated that the environmental conditions (temperature, humidity, ventilation rate and carbon dioxide concentration) are not independent of each other, although each may have its own optimum for hatchability and chick quality. The optimum for each incubation factor may alter differentially according to the starting material, the incubating egg. There is also some doubt as to whether maximal hatchability is always the best indicator for other parameters such as chick quality and/ or post-hatching variability, even if optimal hatchability is a prerequisite for successful incubation. In view of some recent physiological findings, together with new opportunities for technical control (more independent control as well as more control over the variability in the classical physical conditions in the incubator) to improve hatchability, kurtosis of hatching and chick quality should be considered.
^ Top page
.
REFERENCES
ABD EL AZIM, A. (1991) Effect of periodic cooling and warming of incubating chicken eggs on hatchability, postnatal growth and heat stress tolerance. PhD Thesis, Cairo University
ANCEL, A. and VISSCHEDIJK, A.H.J. (1993) Respiratory exchanges in the incubated egg of the domestic guinea fowl. Respiration Physiology 91: 31-42
ANDE, T.B and WILSON, H.R. (1981) Hatchability of chicken embryos exposed to acute high temperature stress at various ages. Poultry Science 60: 1561-1566
AVARUTINA, A.J. and KISLJUK,S.M. (1982) Formation of the reaction of hypothalamic-hypophyseal-adrenocortical system to cooling during chick embryogenesis. Ontogenesis 13: 404-408
AVARUTINA ,A.J., GALPERN,I.L. and KISLJUK, S.M. (1985) Stimulation of adrenals during the critical periods of development and production in folks. World’s Poultry Science Journal 41: 108-114
BAROTT, H.G. (1937) Effects of temperature, humidity and others factors on hatch of eggs and on energy metabolism of chick embryos. US Departmental Agricultural Technical Bulletin 553: 1-45
BRIAN, H. (2000) Incubation, the physical requirements. International Hatchery Practice 14: 25
BUTLER, D.E. (1991) Egg handling and storage at the farm and the hatchery. In: Avian Incubation (Tullett,S.G.,Ed.) Butterworths, London, pp. 195-203
BUYS, N., DEWIL, E., GONZALES, E. and DECUYPERE, E. (1998) Different CO2 levels during incubation interact with hatching time and ascites susceptibility in two broiler lines selected for different growth rate. Avian Pathology 27: 605-612
CRITTENDEN, L.B. and BOHREN, B.B. (1962) The effects of current egg production, time in production, age of pullet, and inbreeding on hatchability and hatching time. Poultry Science 41: 426-433
DECUYPERE, E. (1984) Incubation temperature in relation to postnatal performance in chickens. Archiv fur Experimentalle Veterinarmedizin 38: 439-449
DECUYPERE, E. and MICHELS, H. (1992) Incubation temperature as a management tool: a review. World’s Poultry Science Journal 48: 27-38
DECUYPERE, E., NOUWEN, E.J.,KUHN, E.R., GEERS, H. and MICHELS, H. (1979) Indohormones in the serum of chick embryos and pos-hatching chickens as influenced by incubation temperature. Relationship with the hatching process and thermogenesis.Annual de Biologie Animal et Biochemie Biophysique 19: 1713-1723
DECUYPERE, E., IQBAL, A., MICHELS, H., KUHN, E.R., SCHNEIDER, R. and ABD EL AZIM, A. (1988) Thyroid hormone response to thyrotropin releasing hormone after cold treatment during pre- and postnatal development in the domestic fowl. Hormone and Metabolism Research 20: 484-489
DEEMING, D. C. (1989A) Characteristics of unturned eggs: critical period, retarded embryonic growth and poor albumen utilisation. British Poultry Science 30: 239-249
DEEMING, D. C. (1989B ) Importance of sub-embryonic fluid and albumen in the embryo’s response to turning of the egg during incubation. British Poultry Science 30: 239-249
DEEMING, D. C. (1990) Turning helps hatchability. Poultry Misset 4: 27
DEEMING, D. C. (1991) Reasons for the dichotomy in egg turning in birds and reptiles. In: Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles( Deeming D.C. and Ferguson W.J., Eds), Cambridge University Press, Cambridge, pp. 307-323
DEEMING, D. C. (1996) Large eggs: an incubation challenge. Poultry International 35: 50-54
DIBNER,J. (1999) Feeding hatching poultry: avoid any delay. Feed International December 1999:30-34
FUNK, M.E. and IRWIN, M. R. (1955) Hatchery Operation and Management. John Willey & Sons Inc., New York
HAQUE, M.A., PEARSON, J.T., HOU, P.-D.L. and TAZAWA, H. (1996) Effects of pre-incubating egg storage on embryonic functions and growth. Repiration Physiology 103: 89-98
IQBAL, A., DECUYPERE, E. KUHN, E.R. and ABD EL AZIM, A. (1989) Plasma iodohormone concentrations in early and late hatched chicks incubated at different temperatures. Medical Science Research 17: 269-270
IQBAL, A., DECUYPERE,E. ABD EL AZIM, A. and KUHN, E.R. (1990) Pre- and pos-hatch high temperature exposure affects the thyroid hormones and corticosterone response to acute heat stress in growing chicken (Gallus domesticus). Journal of Thermal Biology 15: 149-153
KUHN, E.R., DECUYPERE, E., COLEN, L.M. and MICHELS, H. (1982) Post hatch growth and development of circadian rhythm for thyroid hormones in chicks incubated at different temperatures. Poultry Science 61: 540-549
LAPAO, C., GAMA, L.T. and SOARES, M.C. (1999) Effects of broiler breeder age and lengh of egg storage on albumen characteristics and hatchability. Poultry Science 78: 640-645
McLOUGHLIN, L. And GOUS, R.M. (1999) The effect of egg size on pre- and post-natal growth of broiler chickens. World Poultry 15: 34-38
MEIJERHOF, R. (1994) Theoretical and empirical studies on temperature and moisture loss of hatching eggs during the pre-incubation period. PhD Thesis, Landbuowunicersiteit te Wageningen, The Nederlands
MERRIT, E.S. and GOWE, R.S. (1965) Post embryonic growth in relation to egg weight. Poultry Science 44: 477-480
MINNE, B. and DECUYPERE, E. (1984) Effects of late prenatal temperatures on some thermoregulatory aspects in young chickens. Archiv fur Experimentalle Veterinarmedizin 38: 374-383
MIROSH, L.W. and BECKER, W. A. (1974) Storage and incubation temperature effects on hatching time of coturnix quail eggs. Poultry Science 53: 432-434
MORAN JR, E.T. (1990) Effects of weight, glucose administration at hatch, and delayed access to feed and water on the poult at 2 weeks of age. Poultry Science 69: 1718-1723
MUAMBI, S., DECUYPERE, E. and MICHELS, H. (1980) Influence de la duree de conservation des oeufs sur la duree d’incubation, le taux d’eclosion et la croissance postnatale chez la rate de volaille “Rhode Island Red”. Reveu Zairose des Sciences Nucleaires 1: 65-83
MUAMBI, S., DECUYPERE, E . and MICHELS, H. (1982) Influence du stockage des oeufs a couver sur la duree d’incubation et le taux serique d’hormone thyroidiennes chez l’embryon du poulet. Reprodution, Nutrition, Developm,ent 21: 585-589
NOY, Y. and SKLAN, D. (1999) Energy utilization in newly hatched chicks. Poultry Science 78: 1750-1756
NVOTA, J., VYBOH, P., JURANI, M., LAMOSOVA,D.,BODA,K and BAROSKOVA,Z. (1980) The influence of environment in the early ontogenesis on the development of endocrine functions and body growth in fowls. Proceedings of the 28th International Congress of Physiological Science ,p.616A
OKUDA, A. and TAZAWA, H. (1988) Gas exchange and development of chicken embryos with widely altered shell conductance from the beginning of incubation. Respiration Physiology 74: 187-198
PEEBLES, E.D. and BRAKE, J. (1986) The role of the cuticle in water vapor conductance by the eggshell of broiler breeders. Poultry Science 65: 1034-1039
PINCHASOV, Y. and NOY, Y. (1993) Comparision of post-hatch holding time and subsequent early performance of broiler chicks and turkey poults. British Poultry Science 34: 111-120
RAGHAVAN, V. (1999) Give day-old chicks the best start. World Poultry 15: 28-29
ROMANOFF, A. L. (1935) Influence of incubation temperature on the hatchability of eggs, postnatal and growth and survival of turkeys. Journal of Agricultural Science 25: 318-325
ROMANOFF,A.L. (1936) Effects of different temperature in the incubator on the prenatal and postnatal development of the chick. Poultry Science 15: 311-315
SHANAWANY, M. M. (1992) Identifying the causes of hatching failure. World Poultry 8: 57-59
STURKIE, P. D. and WILLIAMS, A. G. (1945) Studies on pregatrular development, early embryonic development and hatchability of prematurely laid eggs of the hen. Poultry Science 24: 546-554
TAYLOR, G. (1999) High-yield breeds require special incubation. World Poultry 15: 27-29
TAZAWA, H. (1980a) Oxygen and CO2 exchange and acid-base regulation in the avian embryo. American Zoology 20: 395-404
TAZAWA, H. (1980b) Adverse effect of failure to turn the avian egg on embryo oxygen exchange. Respiration Physiology 41: 137-142
TAZAWA, H. and WHITTOW, G. C. (2000) Incubation physiology. In: Sturkie’s Avian Physiology (Whittow G.C., Ed.), Academic Press, London, pp. 617-634
THOMPSON, J.B., WILSON, H.R. and VOITLE, R.A. (1976) Influence of high temperature stress of 16-day embryos on subsequent hatchability. Poultry Science 55: 892-894
TULLET, S.G. (1990) Science and the art of incubation. Poultry Science 69: 1-15
TULLET, S. G. and BURTON, F.G. (1996) The recent reawakening of interest in bird physiology particularly eggs. eggshell porosity and embryonic respiration. Wissenschaftliche Zeitschrift der Humbolt-Universitat zu Berlin, Mathematisch-Naturwissenschaftliche Reihe 35: 273-284
VIEIRA, S.L. (1999) Feeding the newly-hatched broiler chick. World Poultry15: 17-18
VIEIRA, S.L. and MORAN Jr, E.T. (1999) Effects of age of origin and chick post-hatch nutrition on broiler live performance and meat yields. World’s Poultry Science Journal 55: 125-142
WILSON, H.R. (1991) Physiological requirements of the developing embryo: temperature and turning. In: Avian Incubation (Tullett, S.G.,Ed.), Butterworth-Heinemann, London, pp. 145-156
If you need to download this article, please do not hesitate to contact us!

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam