.
...
.
VECTORMUNE® ND- NEW CONCEPT FOR REDEFINING ND VACCINATION PROGRAM
By Pascal PAULET - Poultry Corporate Product Manager, Ceva Santé Animale, France
.
Newcastle disease has again become a first priority concern in recent years in different part of the world. Despite being recognised 87 years ago, conventional vaccines have limitations which mean that veterinarians struggle to control the disease, even today.
Newcastle so far was controlled through the usage of live or killed vaccines.
.
The live vaccines induce good local and general immunity but they need to be applied several times and they have to replicate in the birds organs. This can create lesions that can impact the flock performances. Moreover, the live vaccines can spread from vaccinated birds to non vaccinated birds creating rolling infections on the farms. But the main roadblock is the limitation of the vaccine take when the vaccine is applied at the hatchery with chicks which have a high level of maternally derived antibodies.
With inactivated Newcastle vaccines the immunity is long lasting but the maternally derived antibodies interaction when the vaccine is applied at the hatchery is still present.
VECTORMUNE® ND is a Ceva innovative vaccine that protects against both Newcastle and Marek’s disease. VECTORMUNE® ND is sold in 25 countries around the world and with more than 2 billion birds vaccinated in 2012 the vaccine value has been extensively evaluated by poultry producers in different field conditions.
VECTORMUNE® ND is able to bring to the industry strong advantages in both low and high challenge countries for Newcastle disease. Because maternally derived antibodies do not affect its efficacy, VECTORMUNE® ND can be given at the hatchery, in ovo or at day of age. Based on a HVT vector it provides homogenous lifelong protection for every chicken vaccinated.
Moreover VECTORMUNE® ND is extremely safe. It does not replicate in the respiratory tract and does not induce post vaccination reaction.
Each vector vaccine is unique by the techniques and steps of its development. Ceva R&D has created a vector vaccine, based on a well known HVT vaccine (strain FC126) where the Fusion protein gene from a lentogenic ND virus has been inserted in.
A very specific attention has been paid in order to not impact HVT replication capacity : through the patented insertion site (in between 2 genes UL 45& UL 46) and to obtain a high level of gene expression to get a high level of stimulation of the bird’s immune system with a patented promoter (pec) that induce.
Therefore due to these specificities VECTORMUNE® ND replicates and expresses strongly the F gene in order to induce a high level of protection. VECTORMUNE® ND induces an early onset of immunity and a strong control of challenge virus and a high reduction of challenge virus shedding compare that make its uniqueness.
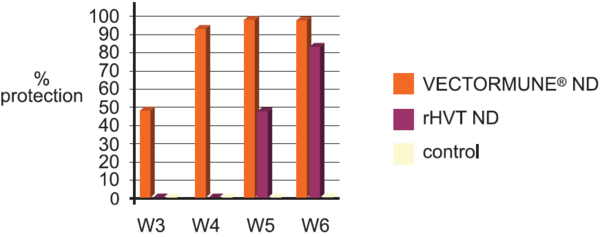
A comparison trial has been conducted in a laboratory to measure the impact of the construction technic of the vaccine on the onset of immunity.
Commercial Broilers received either VECTORMUNE® ND or a rHVT ND at day 1 SQ, and received on a regular basis a ND velogenic challenge virus at 5.0 ELD 50 / bird per intranasal route.
VECTORMUNE® ND induces a earlier onset of immunity and a higher level of protection after single administration at day 1 compare to the test vaccine.
Produced at the new state-of-the-art facility at the Ceva Biomune Campus in Kansas, VECTORMUNE® ND is proving to be a major step forward in Newcastle protection around the world.
.
(Source: "Axis Magazine - Poultry” - VIV Asia 2013 Edition)

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam